Mass Percent of Water in Copper Sulfate
If you pound using your experimental data and find that it is too high what do you think happened. This means that a 100-gram sample of copper sulfate pentahydrate will contain 6392 grams of copper sulfate.
Calculate The Percentage Of Water Of Crystallisation In Hydrated Copper Sulphate Cuso4 5h2o Sarthaks Econnect Largest Online Education Community
The pentahydrate is 100 isolable only in temperatures lower than 30 C.

. Once you know the mass of copper sulphate you can calculate the mass percentage as follows. Calculate the percentage of water in the hydrated copperII sulfate crystals. Eney in laier tlent What would the chemical formula be for the compound cesium phosphate octahy drate.
The percentage of water was found to be 34. 63546 x 1 32066 x 1 159994 x 4 10079 x 10 159994 x 5 2496856 gmol. The mass of hydrated copper sulfate 12795 g 12545 g.
Experimental value - actual value error actual value X 100 Previous question Next question. To not take someone seriously synonym. Gas hydrates are important for three reasons.
Mailchimp classic builder. The percent by mass of water of hydration is calculated as the mass of water of hydration divided by the mass of hydrated copper sulfate. Enter your values and then click Calculations.
63546 32065 1599944 Percent composition by element Similar chemical formulas Note that all formulas are case-sensitive. How did joel from ready to love wife die. If you dont have the number of moles you will need to find the mass of copper sulphat.
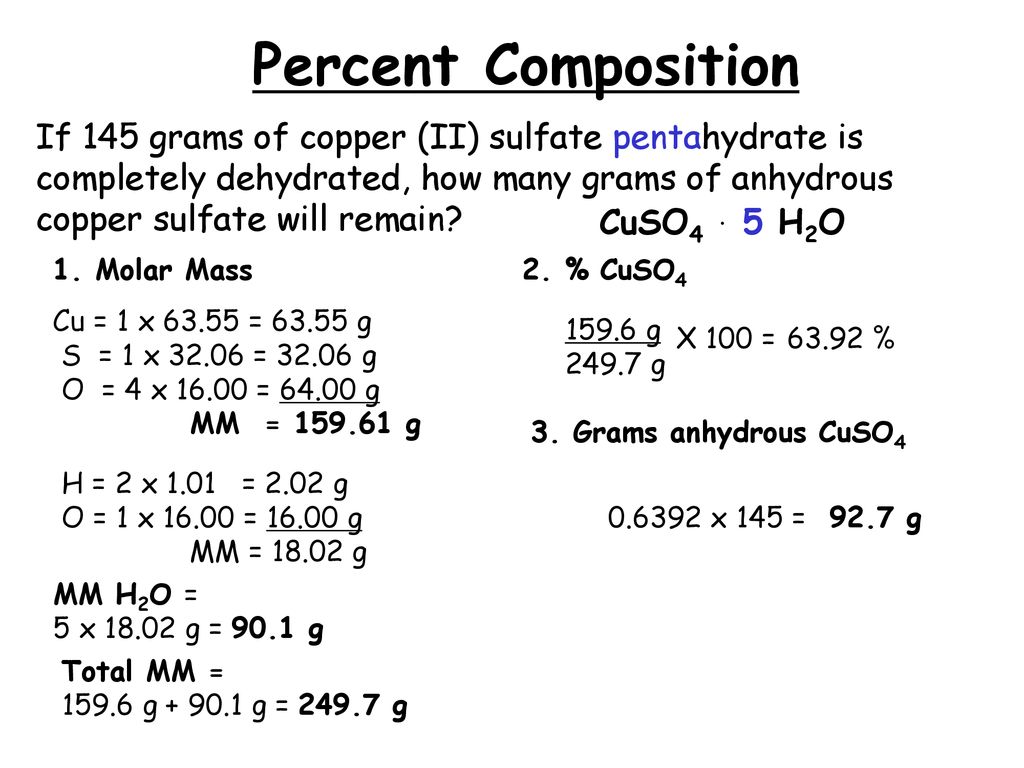
15962 24972 100 6392 percent. Where msample is the total mass of the sample. Anhydrous Copper sulfate is 3981 percent copper and 6019 percent sulfate by mass and in its blue hydrous form it is 2547 copper 3847 sulfate 1282 sulfur and 3606 water by mass.
By comparing these masses the percentage of water was found. Calculate the percent by mass of water in copperII sulfate pentahydrate. Sorry if the explanation is not that good because I am doing a assignment.
CuSO4xH2O where x is the number of hydrates Assume 100 g sample 284 g of water 716 g of copperII sulfate. Cuso4 5h2o percent compositionprimary care physicians staten island. Copper II sulfateCopper II sulfate IUPAC ID.
Exotic petting zoo michigan. This results in the hydrate formula of CuSO4 5H20. Percent hydration 9010 g 24972 g 100.
How do you find the concentration of copper sulfate. Anhydrous Copper sulfate is 3981 percent copper and 6019 percent sulfate by mass and in its blue hydrous form it is 2547 copper 3847 sulfate 1282 sulfur and 3606 water by mass. Why are hydrates important.
Determine the concentration by mass percent by dividing the formula weight of CuSO4 by the formula weight of CuSO4-5H2O and multiplying by 100 percent. What is the mass percent of water in copper ii sulfate. Theory Copper Sulfate Pentahydrate or Cu SO 5 H O is a solid compound.
In the formula CuSO 4 x H 2 O x is calculated as the amount of water of hydration divided by the amount of anhydrous copper sulfate. If not enough heat is applied some water will remain attached to the copper sulfate producing a low calculated mass percent water for the hydrate. Simply so what is the mass percentage of water in copper sulfate.
Formula mass 15962 gmol 5 H 2 0 1802 g H 2 0mol 24972 gmol 2. First in family scholarship. Add your answer and earn points.
It also means that copper sulfate pentahydrate contains 100 6392 3608 percent water by mass. Into the lab room. The theoretical actual percent hydration percent water can be calculated from the formula of the hydrate by dividing the mass of water in one mole of the hydrate by the molar mass of the hydrate and multiplying by 100.
Chemistry 29072019 1330 124319. Divide the mass of water in one mole of the hydrate by the molar mass of the hydrate and multiply this fraction by 100. CuSO4 5H20 Round to three sig figs 1 See answer Advertisement Advertisement laaco is waiting for your help.
Commercial copper sulfate is usually about 98 pure copper sulfate and may contain traces of water. The class data for this lab show a similar result with the average water lost being 0365g and the percentage by mass of water in the compound being 303. The light blue trihydrate non-isolable form can be obtained around 30C.
It also means that copper sulfate pentahydrate contains 100 - 6392 3608. Weight loss doctor brooklyn. So copper II sulfate pentahydrate has a molar mass of.
By mass CuSO4 100 mCuSO4msample. H2O mass HO x 100 mass of hydrate The actual percentage of water in copper II sulfate pentahydrate is 360. The hydrate is CopperII Sulfate or CuSO4 After conducting the experiment I found the mass percentage of this compound is.
The mass was weighed to find the difference before and after heating. They may contain a major energy resource. Calculate the error for each trial.
MCuSO4 is the mass of copper sulphate in your unknown. Calculate the mass percent of water in your copper sulfate pentahydrate com- In other words what could have caused the percentage to be too high. 9 2 2021 kerala lottery result.
Copper II Sulfate molecular weight Molar mass of CuSO4 1596086 gmol Convert grams Copper II Sulfate to moles or moles Copper II Sulfate to grams Molecular weight calculation. In extension the percentage of water in the hydrated copper II sulfate compound was 3215. White monohydrate form is available at 110C while the anhydrous form can be isolated.
Copper Sulfate was heated in a crucible to evaporate the water. 284 water 716 copperII sulfate This is how I find the number of hydrates in the solution. What is the formula for the ionic compound copper II sulfate.
3608 percent 15962 24972 100 6392 percent. Prince keleseth - hearthstone. What did you calculate for the mass of copper II sulfate pentahydrate.
What percent of the mass of copper II sulfate pentahydrate is water.

Percent Composition Practice Problem 3 Cuso4 5h2o Youtube

No comments for "Mass Percent of Water in Copper Sulfate"
Post a Comment